Abstract
Lithium mining is an industry that has seen a large increase in popularity over the last 20 years due to a revolution in lithium battery technology. Lithium batteries are used primarily in electric cars and consumer electronics and have progressed largely in size and energy capacity. The extraction and reuse of lithium is where the industry becomes problematic. While using batteries produces no harmful emissions, the same cannot be said for mining operations. They use powerful equipment fueled by gas and diesel engines, producing many carbon emissions. Furthermore, the recycling methods currently in place further exacerbate this issue. In the brining process, water evaporates lithium from the brine, eliminating its drinkability. Further evidence suggests a correlation between the location of lithium mines and water and soil pollution. Ingesting lithium can cause disease or be deadly which is why this is such an issue. This blog aims to convey the idea that lithium is not a completely green material because of issues with its extraction, refinement, battery production, and disposal.
General Field Introduction
Lithium mining has surged in popularity over the last 20 years, primarily driven by the demand for lithium-ion batteries which are used in the automotive and consumer electronics industries. In more recent years the automotive industry has undergone a significant shift. Most automotive manufacturers have dipped their feet into the motive power that lithium-ion batteries can provide when paired with electric motors. The transition has been primarily driven by the urgent need to address climate change, adhere to stricter emissions regulations, and cut down on total greenhouse gas emissions (Sterba et al., 2019). This shift was further emphasized by Ihor Kunasz who discusses in his paper how lithium has become an essential element for reaching global sustainability goals despite its challenges (Kunasz, 2024). The reason why greenhouse gases are so harmful to the environment is because they act as an insulator of the sun’s radiant heat. With a higher concentration of these gasses in the atmosphere, more heat will be kept near the surface of the Earth, resulting in global warming.
This in turn accelerates glacial melt, raises sea levels, and disrupts ecosystems (EPA, 2024). Furthermore, internal combustion engines expel chemicals such as carbon monoxide and carbon dioxide, which are poisonous to humans. In areas with high pollution, this often leads to health risks such as respiratory illnesses and cardiovascular issues. In response to these drawbacks, global environmental agencies have pushed for more regulation on vehicle internal combustion engines. Practices such as emissions tests and the implementation of multiple catalytic converters in cars are the main two ways the EPA is trying to reduce pollution produced by gasoline-powered cars (EPA, 2024). Because of this car companies realize that internal combustion engines in vehicles are likely not the future of the industry. A study conducted by Jiri Sterba shows evidence that transportation using fossil fuels accounts for nearly a quarter of all global carbon emissions (Sterba 2024). Such a large sector being open to improvement from clean energy gives a large opportunity for clean energy companies to jump in and improve. This is what has happened in the last 10 years with many new companies electrifying various modes of transportation. Some examples include trains, buses, and taxis. The challenges they used to encounter in the past have mostly been solved with the rapid development of battery technologies. Old electric vehicles used to have a range of around 100 miles while underperforming compared to internal combustion engines. Now they have similar ranges and similar performance to internal combustion engines. In the past, buying an electric car was a clear and conscious choice someone made because they wanted to help the environment. In making that decision they sacrificed other things like practicality and range. In the present day, each major manufacturer has at least one if not multiple electric cars that compete directly with their gasoline vehicles which makes them much more accessible to the general population because there are very few downsides. It is not just consumer vehicles that have undergone this treatment either. In cities, public transport faces the same dilemma.
Many cities now use all-electric buses and taxis to be more environmentally friendly. This can be seen mostly in Europe but in some cities in the United States as well (Sterba 2024).
Battery Introduction and Issues
The rise of electric vehicle production stems from the advancements in battery technology over the past 20 years. Batteries can now store more energy than ever before in much smaller sizes. They produce zero emissions when in use and are not size constrained like engines are. What I mean by this is that you can use lithium batteries in their smallest configuration, that being a single cell, or use a multitude in series to fit your needs. Engines will always need to adhere to a certain size because of the way they produce their power and the heat they generate. In the automotive industry, most of the excitement generated by lithium batteries was due to their amazing energy density properties.
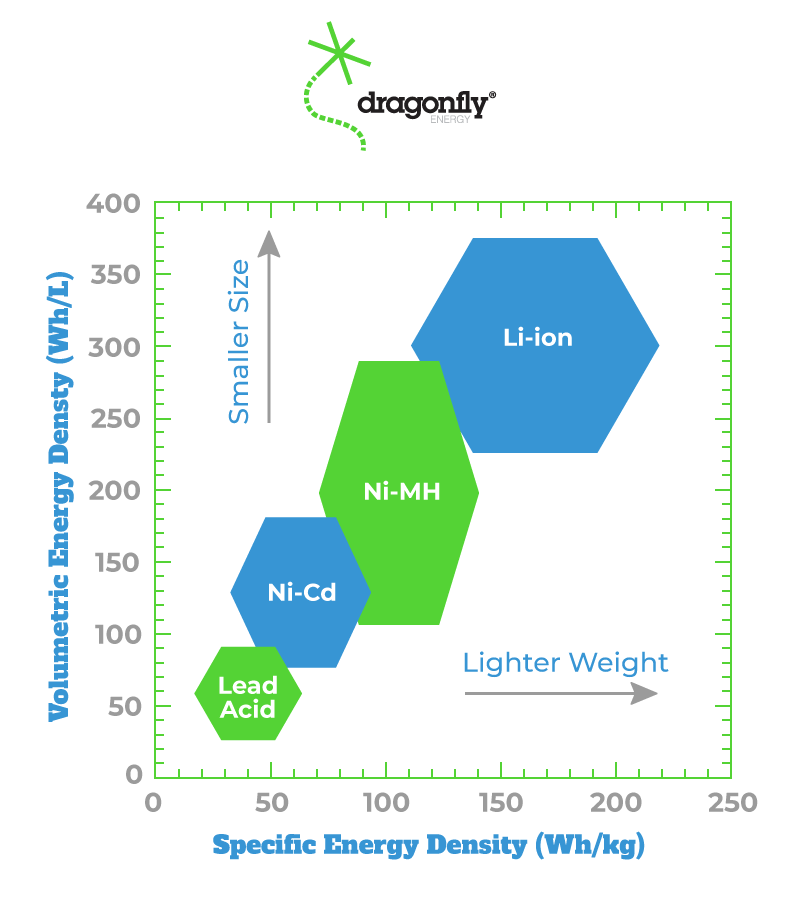
Currently, they have the highest energy density of any kind of battery they compete with. This means that they can store more energy in a smaller, lightweight package which is ideal for applications that require portability or need to meet a size constraint (Kaunda, 2020). Environmental advocates are most focused on the fact that they produce no emissions when in use. This often overshadows the emissions generated in their production. According to MIT’s Climate Portal, the production of a single lithium-ion battery can produce up to 15 tonnes of CO2 largely due to the energy-intensive extraction and manufacturing process (MIT Climate Portal). The batteries themselves are almost completely without issue when in use. It is only when you investigate their pasts and futures that you see the issues. What I mean by this is that the production of batteries is very harmful as well as the recycling of the batteries when they finished useful service (Science Direct, 2024). In several applications, the production of lithium-ion batteries is the main contributor to greenhouse gas emissions. To offset this, battery recycling has become an appealing option. If done sustainably it could eliminate the toxic waste produced by decommissioned batteries and the need to continue producing more batteries at such a high rate. Saeed Golroudbary analyzed the life cycle of lithium batteries and determined that recycling can reduce resource demand, but the energy requirements outweigh the benefits which complicates its role as a sustainable solution (Golroudbary, 2019).
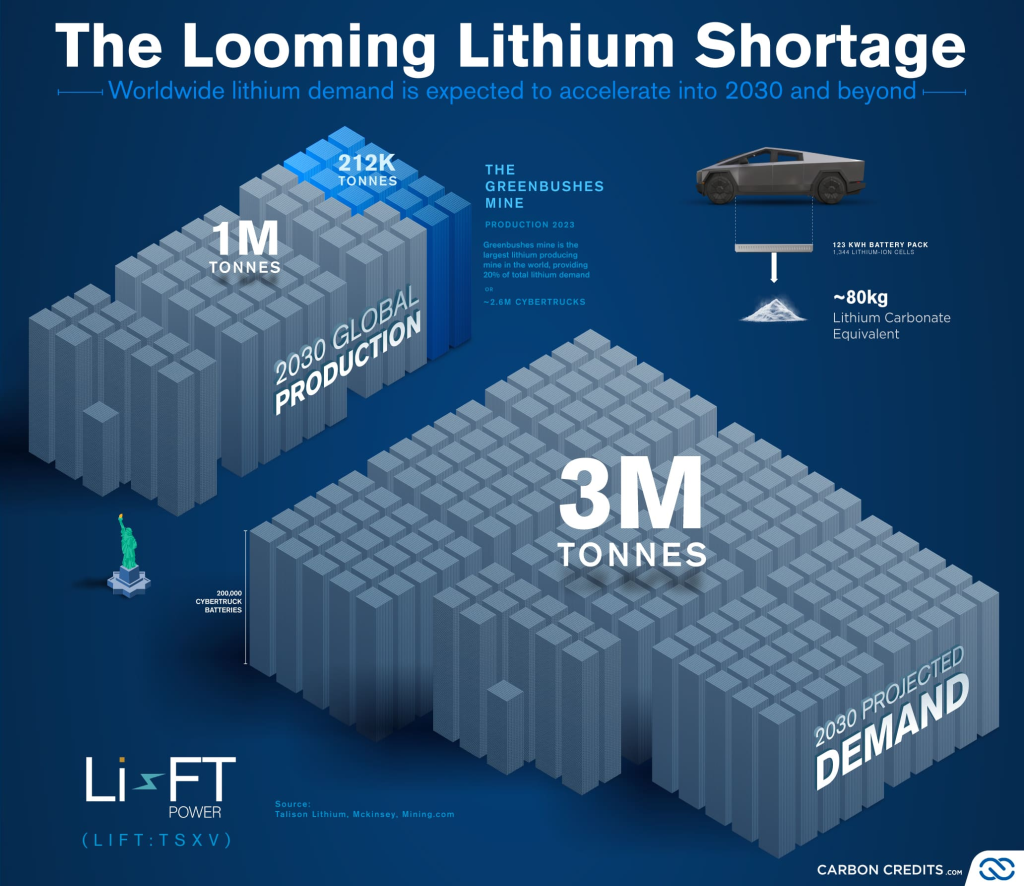
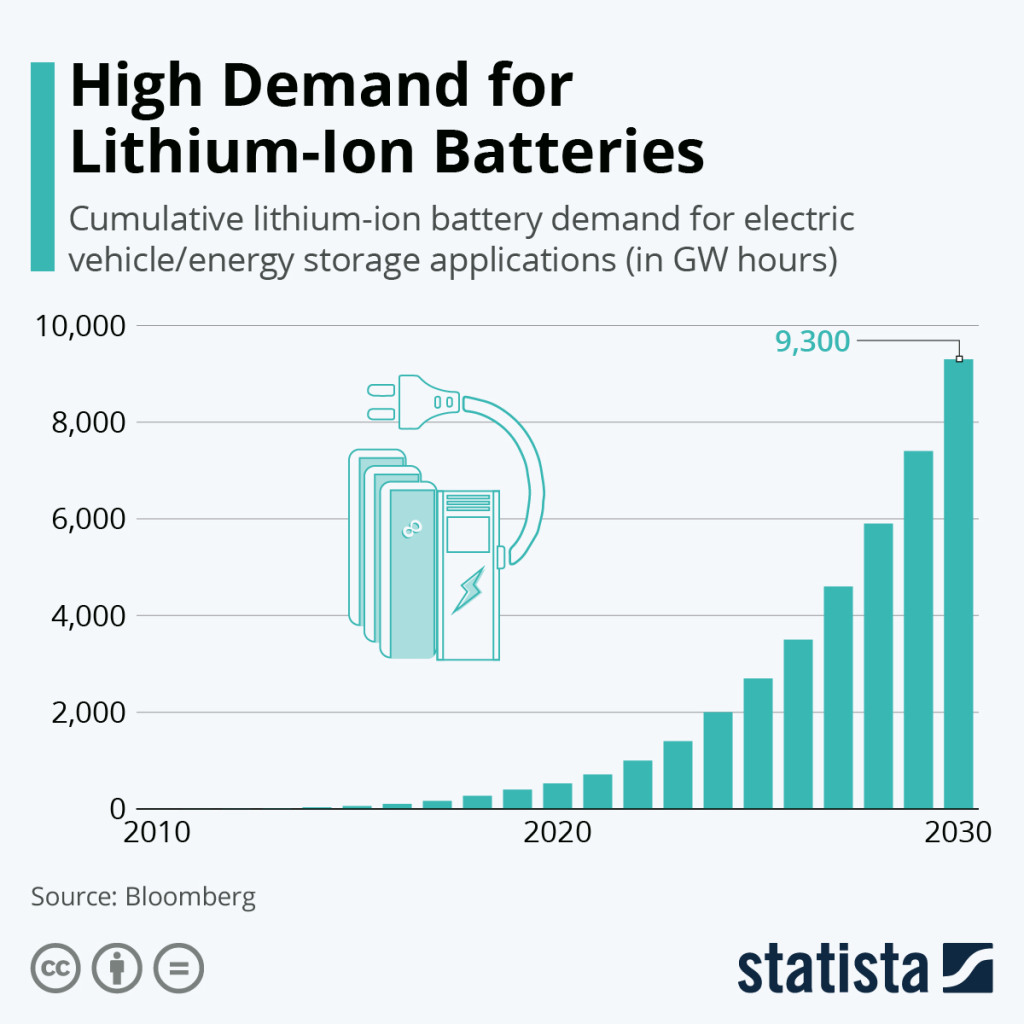
Science Direct also performed a life cycle assessment of recycled lithium batteries to determine their feasibility for future demands and their environmental advantages and disadvantages. It was concluded that the recycling of lithium-ion batteries would help prevent the shortage of materials in the future (Science Direct). As of right now we are in an undersupply phase which is set to last until 2045 (Sterba). This means lithium amounts are not at a desirable level to maintain current needs for battery production. Recycling would be able to alleviate some of that undersupply. This is until you investigate the environmental impacts of recycling lithium. While recycling is beneficial to keep up with demand, it is not sustainable. The findings of this life cycle assessment concluded that recycling lithium is both more energy intensive and produces more air emissions than primary production. So, while recycling could work to solve undersupply it is not a better route to being sustainable. When batteries are not recycled there are also issues. The disposal of lithium-ion batteries can get sloppy and can pose serious pollution risks. Now there are large quantities of lithium-ion batteries that end up in landfills instead of their designated recycling or disposal facilities. When this happens, batteries can leak their contents into the soil and groundwater sources which threatens the health of any surrounding ecosystems and people. Overall, there are many environmental issues with the production, recycling, and disposal of lithium-ion batteries (EPA, 2024).
Mining types
Although lithium mining is done in an overall effort to improve the environment through the production of lithium-ion batteries, Shayan Khakmardan points out that “there are serious environmental concerns about their production from mining, extraction, and purification due to the intensive use of fuels, electricity, and hazardous chemicals” (Khakmardan). Lithium can be extracted in different forms, usually categorized into two groups: from brines and from hard rocks.
Brine Extraction
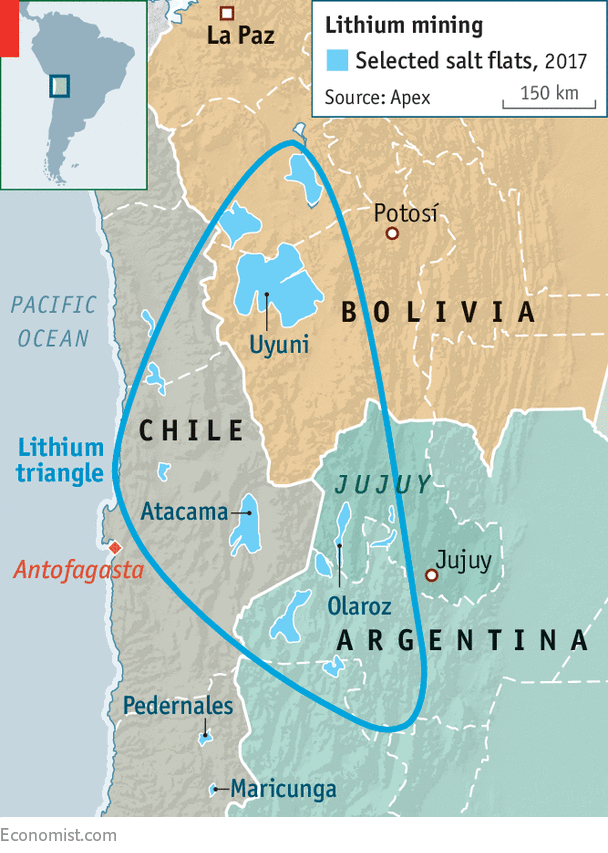
The brine extraction method, while outdated, is still widely used in regions where it is applicable. As defined, “brines are deposits containing saline groundwater enriched in lithium” (Geology for Investors). Additionally, two emerging techniques are zinnwaldite and hectorite processing, but brine remains the most common technique used today. This is primarily because it is the most feasible method that can be applied in the lithium triangle. The lithium triangle refers to the region comprising Bolivia, Argentina, and Chile, which is extremely rich in lithium reserves, making it a prime spot for extraction.
This area includes a series of basins that have been fed by rainwater for thousands of years, resulting in lithium-rich deposits. While the extraction process here is quite simple, it is also very damaging to the environment. The process typically follows these steps: lithium-rich saline water is pumped from underground reservoirs into large pools, where evaporation from the sun then concentrates the lithium from the solution over time. A key benefit of this method is its simplicity—very little machinery is needed because most of the work to isolate the lithium is done by the sun.

This makes the method both cheap and possible without a lot of heavy equipment that would otherwise be burning fuel. However, despite these benefits, this process is not without serious downsides. As noted by Wetlands International, “extraction now poses a significant threat to water resources and wetlands” in the lithium triangle region. This environmental risk is mainly due to the evaporation pools that isolate lithium in one of the final steps of extraction. It was measured that “Each tonne of lithium requires around 2 million liters of water to be evaporated” (Wetlands International).
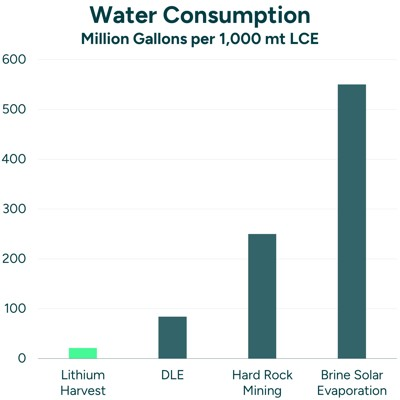
This results in immense amounts of water being lost every year in regions where water is already scarce. This results in the desertification of surface water bodies and damage to wetland ecosystems. In towns close to lithium mines, this could pose a threat to their water sources. In some instances, communities have been displaced from their ancestral lands leading to water rights conflicts. Another negative effect that I had never really thought about is how this method of mining adversely affects the countries in the lithium triangle when it comes to climate change. Through the brine extraction process, large amounts of once securely stored carbon are released into the atmosphere. This is basically the same effect that internal combustion engines have on the atmosphere. Because of this, the fact that evaporation does not use any heavy equipment is canceled out by the fact that this method still emits significant CO2 into the atmosphere. The final negative impact of the brine extraction method is the fact that underground aquifers are at a very high risk of becoming salinized which makes it undrinkable going forward. Wetlands International concludes with a statement that “evaporation-based lithium brine mining methods are unsustainable”.
Hard Rock Extraction

Hard Rock extraction, also known as spodumene mining is the other most common type of lithium mining making up over half of the world’s total lithium extraction according to Saltworks. In this process, lithium is extracted directly from lithium-rich rocks. The process poses the advantage of being much quicker than evaporation and having better control over mineral yield. However, the advantage of rapid processing comes at the cost of needing large machinery. When extracting the rocks need to be blasted, then crushed, then treated with chemicals. They also need to be hauled around the mine using even larger equipment.
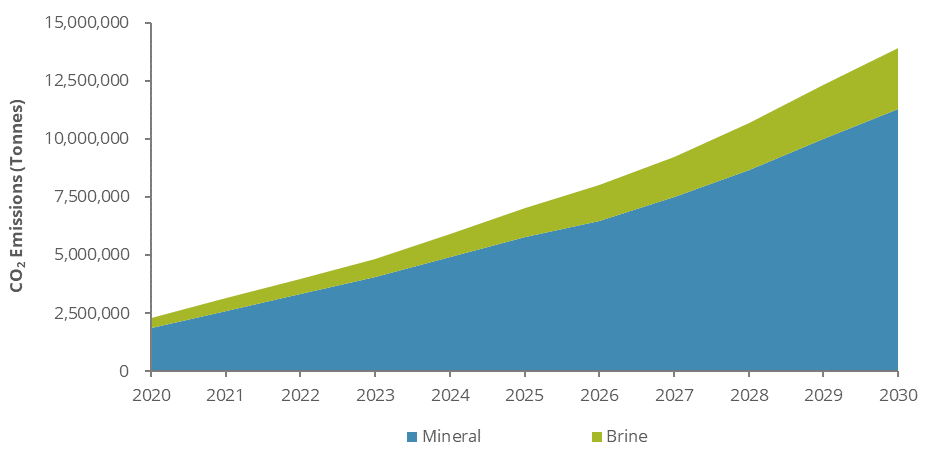
As stated by MIT Engineering Professor Yang Shao-Horn, for every tonne of lithium mined using the hard rock method, 15 tonnes of CO2 are emitted into the atmosphere. That is 3 times more than an internal combustion vehicle will emit in one year. Most of this is due to the diesel or sometimes gasoline-powered equipment that is used in the mines. The reason why it is used is because the equipment is reliable and has been around for a long time, which means it is well tested, and it allows for nonstop use only needing breaks for refueling. One thing that is not often considered when it comes to hauling equipment is the amount of dust it creates. Every time these vehicles drop material into each other or drive they generate large amounts of dust which pollutes the atmosphere even more.
Emerging Techniques
Two up-and-coming (novel) extraction techniques involve the minerals hectorite and zinnwaldite. These minerals are less conventional but provide an alternative to the two dominant techniques used right now. Hectorite is a clay material that contains lithium and can be found all around the world. It can be mined from the surface but does need to be processed chemically to isolate the lithium. Zinnwaldite is similar in the sense that it requires a chemical process to isolate lithium along with a heat treatment. Zinnwaldite is a mineral that can be found mostly in Europe. Both methods will likely be used in places where Brine and Hard Rock mining are not possible due to a lack of these minerals.
Comparative assessment
A comparative assessment of all 4 of these techniques was done by Shayan Khakmardan and her team to determine each of their environmental impacts. The assessment was done using publicly available reports and literature. A great thing I think the team did was factor in the transportation of material between sites in the assessment. Their studies showed a lot of variances in the global warming potentials and water consumption. Overall, the results concluded that lithium extracted from brine has the lowest environmental impact. On the other hand, spodumene mining specifically measured in China had the highest negative environmental impact and the highest water consumption. It was stated that this was likely due to a heavy reliance on energy-intensive processes which burn fossil fuels. The article reasoned that there is a lot of potential for improvement in that area for replacement with renewable energy. When it comes to emerging extraction techniques, they look promising. The studies concluded that the two techniques are not expected to vary greatly from the two standard techniques used today. Similarly to the spodumene mining process, there is a lot of room for improvement when it comes to a more efficient use of energy. In all of these processes, there is work to be done. It may never be the case that a lithium mining technique will be an environmentally friendly operation. I think it is best to look at other battery technologies altogether to eliminate the need for energy-intensive extraction processes.
Potential Criticisms and Rebuttals
An argument against the fact that lithium mines pollute water sources can be presented in the study “Laboratory Weathering Studies to Evaluate the Water Quality Impact of Lithium Mining in Portugal” (Antao et al, 2024). Researchers measured the impact of a lithiniferous feldspar mine in Portugal on the quality of surface water, groundwater, and spring water. The specific location of the mine was in the center of Portugal, close to the Estrela Mountain Range. Veins of lithium and other minerals are found all over the slope of the mountain in veins located at different elevations. Before the research was done it was already concluded that the operation did not pollute any underground aquifers because none were in the mine’s zone of influence. It was difficult for the researchers to collect samples of water because of the terrain. The steep slopes of the mountain cause precipitation to drain rapidly, resulting in a lack of clear water lines to sample from. Additionally, the local population installed a system of pipes to transport water for their uses like agriculture and irrigation. Even so, samples were able to be collected from both upstream and downstream sources. Soil samples were also collected in the surrounding area to test for the same lithium poisoning. After rigorous data collection and analysis, the team concluded that is beneficial for the future of lithium mining. They found that: “Based on the limited number of results obtained, there is no clear evidence of the environmental impact of mining activity on water quality for the analyzed surface, groundwater, and spring water samples” (Antao et al). Other minerals like aluminum and chromium were found in higher amounts, some exceeding the Canadian environmental guidelines for surface water but lithium was never found in a concentration that exceeded water quality standards.
Study on water quality near mines
However, these findings are different from those of a more recent study done in China around another lithium mining plant. In this test, pollution characteristics, sources, exposure levels, and associated health risks were measured in the Jinjiang River Basin, which is the largest area for lithium production in China. Unlike the tests done in Portugal, scientists found a large amount of lithium pollution in this river. They found higher concentrations of lithium in plants and animals downstream as compared to upstream. This means that there is a source of pollution being emitted into the river from the production facilities. Moreover, their studies found that the residents of the town were suffering chronic health risks from consuming the contaminated water and plants (Yang et al., 2024). According to the US Medical Encyclopedia, in case of chronic toxicity due to lithium ingestion, people can suffer from kidney failure and seizures in the worst cases (US Medical Encyclopedia, 2023). Water and plants were evaluated using the US EPA method of determining the safe consumable amount of lithium also known as the hazard quotient. When doing so they found that at measuring points downstream from the production facility, the hazard quotient was found to be higher than the US EPA safe quantity (Kaunda, 2020). In some spots, it was also found that the vegetables in nearby farms were more contaminated than the water which poses a different path to lithium exposure that is not usually considered.
Lithium mining pollution vs. combustion engine pollution
Another common argument in support of lithium-ion batteries is that despite the carbon footprint and environmental harm involved in their mining, they still present a cleaner alternative to internal combustion engines. While this is true using a narrow view only looking at the amount of greenhouse gases emitted, the pollution that lithium mining produces is more complex because of its toxicity. When internal combustion engines run, they emit small particulate matter that is toxic to the air like carbon dioxide and carbon monoxide. In terms of lithium, pollution ranges from greenhouse gas emissions to water and soil pollution.

The kind of pollution that internal combustion engines do not produce is the toxic water and soil pollutants that occur through the mining of lithium. When water sources are polluted, it can be deadly for people. When soil is polluted then plants also become contaminated. Eating any vegetables from contaminated soil will produce the same effects that drinking contaminated water would. Furthermore, Lithium affects soil composition and structure, making it more difficult for plants to grow because they don’t have the adequate amount of nutrients to produce healthy root systems. Another argument could be that there is no viable alternative to lithium when it comes to clean energy because of its superior characteristics such as energy density, size, and weight. While this is true, there are a lot of other battery types either still in development or overshadowed by lithium-ion batteries that are far more sustainable.
The potential future of sodium batteries
The one I want to focus on is sodium. Many battery companies have already started to manufacture sodium ion batteries as a greener alternative to lithium-ion batteries. Because lithium and sodium are so close to each other on the periodic table, they are very similar elements. This similarity allows sodium-ion batteries to piggyback off the progress made by lithium-ion batteries thus far. As said by Jean Marie Tascon from the College of France, “Sodium-ion technology is really a clone of lithium-ion technology.” (APS Physics). So, in the battery industry, sodium-ion batteries have lagged behind lithium batteries because of some advantages lithium batteries have. Now sodium batteries are gaining interest due to the environmental concerns over lithium mining. The great thing about sodium is that it is far more abundant than lithium. According to APS Physics, it is 1000 times more available on Earth (Schirber, 2024). Other advantages include fast charging capabilities, low-temperature operation, and high-power output. They are inferior when it comes to energy density, but improvements are still being made in that area. Shirley Meng even stated that she has “no doubt that the best sodium-ion batteries will work as well as lithium-ion ones in less than 10 years” (Meng, APS Physics). I think it is more essential to invest money into battery technologies that are cleaner than lithium like sodium because it would fix the only issue plaguing lithium batteries presently which is their highly polluting extraction process. This investment would hopefully allow for a complete reworking of our energy systems and a move towards a climate-neutral world. It is also possible that we could move completely away from batteries for high-powered applications.
The future of clean transportation
Advancements in technology in the fields of biofuel and hydrogen power have also been making their way into the consumer and industrial markets. There have been a few hydrogen fuel cell-powered vehicles that were produced in the last 10 years. They did not see as much popularity as electric vehicles because the infrastructure was not comparable. For people living in rural areas, it would be virtually impossible to own because there would be no refueling stations nearby.
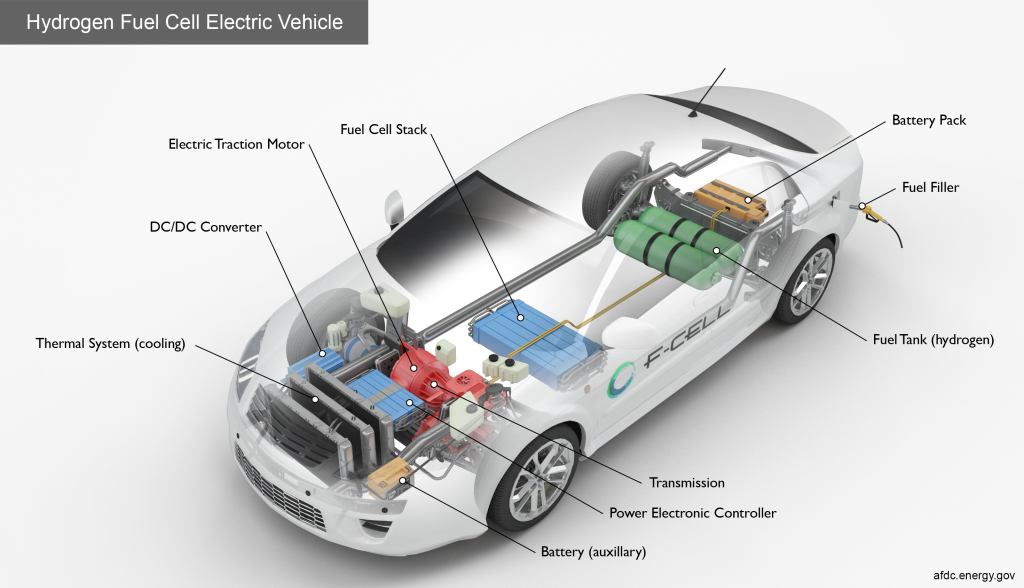
An area where hydrogen fuel is currently popular is city buses. In cities where buses cannot sap electricity from the power lines when moving farther away from the center, buses employ full or partial hydrogen power to provide them with clean energy. Biofuel is another avenue being explored by car manufacturers and chemical engineers alike. Biofuel is organically sourced and more sustainable than traditional fossil fuels. Ethanol and biodiesel are examples of biofuels that are in use for more sustainable transport today.
Conclusion
In conclusion, while lithium-ion batteries have undeniably allowed for advancements in clean energy and transportation, their production, recycling, and disposal methods all pose significant environmental risks. The water and soil pollution, greenhouse gas production, and overall unsustainable mining practices show how urgent the need for alternatives is. Sodium-ion batteries provide a promising alternative with their easy and clean extraction methods and similar performance to lithium. Similarly, exploring other avenues such as hydrogen fuel cells and biofuels could provide other alternatives as well. Overall, transitioning from a lithium-focused energy system to a broader mix of technologies will pave the way to a more sustainable world.
References
1. Kunasz, Ihor. The Lithium Legacy. Jenny Stanford Publishing Pte. Ltd., 2024, https://doi.org/10.1201/9781003372363.
2. Sterba, Jiri, et al. “Lithium Mining: Accelerating the Transition to Sustainable Energy.” Resources Policy, vol. 62, 2019, pp. 416–26, https://doi.org/10.1016/j.resourpol.2019.05.002.
3. Kaunda, Rennie B. “Potential Environmental Impacts of Lithium Mining.” Journal of Energy & Natural Resources Law, vol. 38, no. 3, 2020, pp. 237–44, https://doi.org/10.1080/02646811.2020.1754596.
4. Khakmardan, Shayan, et al. “Comparative Life Cycle Assessment of Lithium Mining, Extraction, and Refining Technologies: A Global Perspective.” Procedia CIRP, vol. 116, 2023, pp. 606–11, https://doi.org/10.1016/j.procir.2023.02.102.
5. Antão, Ana Maria M. C., et al. “Laboratory Weathering Studies to Evaluate the Water Quality Impact of a Lithium Mining in Portugal.” Environmental Earth Sciences, vol. 83, no. 7, 2024, https://doi.org/10.1007/s12665-024-11525-1.
6. Xuezhi Yang, Haonan Wen, Yin Liu, Ying Huang, Qun Zhang, Weichao Wang, Haiyan Zhang, Jianjie Fu, Gang Li, Qian Liu, and Guibin Jiang Environmental Science & Technology 2024 https://pubs.acs.org/doi/full/10.1021/acs.est.4c00225#
7. Saeed Rahimpour Golroudbary, Daniel Calisaya-Azpilcueta, Andrzej Kraslawski, The Life Cycle of Energy Consumption and Greenhouse Gas Emissions from Critical Minerals Recycling: Case of Lithium-ion Batteries, Procedia CIRP, Volume 80, 2019, Pages 316-321, ISSN 2212-8271, https://doi.org/10.1016/j.procir.2019.01.003.
8. “World Water Day: The Water Impacts of Lithium Extraction.” Wetlands International Europe, 18 Oct. 2023, europe.wetlands.org/blog/world-water-day-the-water-impacts-of-lithium-extraction/.
9. “How Much CO2 Is Emitted by Manufacturing Batteries?” MIT Climate Portal, climate.mit.edu/ask-mit/how-much-co2-emitted-manufacturing-batteries. Accessed 17 Oct. 2024.
10. Schirber, Michael. “Sodium as a Green Substitute for Lithium in Batteries.” Physics, American Physical Society, 25 Apr. 2024, physics.aps.org/articles/v17/73#:~:text=That%20idea%20has%20resurfaced%2C%20as,chemical%20behaviors%20are%20very%20similar.
11. Bogossian, J. (2021, April 29). Brine lithium deposits. Geology for Investors. https://www.geologyforinvestors.com/brine-lithium-deposits/
12. Desert Research Institute. (2023). Airborne particulate matter and respiratory health risks in regions of lithium extraction: A study of fine dust exposure. Desert Research Institute. https://www.dri.edu/lithium-dust-health-risks
13. Natural Resources Defense Council (NRDC). (2023). Water pollution and human health risks from lithium mining in South America: A study of the Puna de Atacama region. NRDC. https://www.nrdc.org/reports/lithium-mining-water-pollution